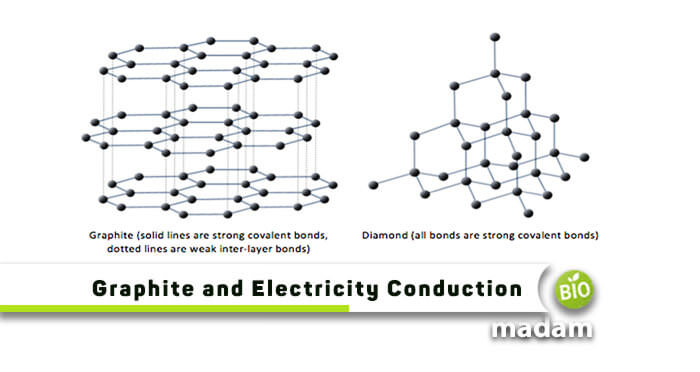
Why Does Graphite Conduct Electricity but Diamond Doesn't
Graphite is one of the very common allotropes of carbon. It is also the most stable allotrope of carbon and thus used in electrochemistry as the standard state for defining the heat of formation of carbon compounds.
Is Graphite A Good Conductor Of Electricity Biomadam
Graphite was accidentally synthesized.

. Graphite is a good conductor of heat and electricity with a density of 209223 gcm 3.
How Does Graphite Conduct Electricity Even If It Is Bonded With Three Carbon Atoms In Which One Of The Bond Is Double Therefore It Is Chemically Stable How Does Free Electron Come

Why Does Graphite Conduct Electricity Bbc Science Focus Magazine
32 Why Graphite Conduct Electricity But Diamond Doesn T While Both Are Made Up Of Carbon
No comments for "Why Does Graphite Conduct Electricity but Diamond Doesn't"
Post a Comment